The market of more than one billion people in China, the extensiveness and convenience of self-testing, if the country in increasing the simultaneous screening of antigens and nucleic acids, the capacity of this market speaks for itself, but at the same time will encounter many challenges, such as the new crown antigen detection reagents required for raw materials and production capacity issues. The price of raw materials, supply cycle, quality, etc. will require companies to make the necessary assessments to reduce the supply risk, here the main analysis of the new crown self-test antigen reagents, one of the raw materials preservatives:
At present, the main preservative used inside the new crown antigen self-test reagent is used in the sample dilution buffer, and the more traditionally used preservative is sodium azide*. As a widely used preservative raw material in the in vitro diagnostic industry, it inhibits bacterial growth, is easily soluble in water, and is suitable for use in most neutral and alkaline environments, and will release toxic HN3 (hydrogen azide) when it meets acid. Its dosage is generally 0.1%~0.5%, which is effective in most cases, but since the azide bond interferes with a variety of enzymes such as horseradish peroxidase (HRP), superoxide dismutase, catalase, etc., reagents containing HRP are generally not recommended. Similarly, it is generally not used for specimen preservation in antibody and enzyme immunoassay experiments. In addition, it can block the electron transfer chain of cells and is toxic to many organisms, so it is also not suitable for in vivo experiments.
Meanwhile, sodium azide* is similar to qingide and is a highly toxic compound with LD50 of 27 mg/kg (rat, oral). It has an inhibitory effect on cytochrome oxidase and other enzymes, and can block the formation of oxyhemoglobin in vivo and has a significant hypotensive effect. Acute poisoning showed dizziness, headache, general weakness, decreased blood pressure, bradycardia and coma. In addition to eye and skin irritation, it can also cause death by inhalation, oral or dermal absorption, and can explode strongly when exposed to high heat or severe vibration, it is both explosive and highly toxic, so great attention should be paid to safety when using it. For safety reasons, the use of sodium azide* is increasingly restricted. In daily use, there are various restrictions on purchase, transportation, storage and use.
This product has another limitation of use in foreign countries, because some foreign hospitals have a long history, some of the downpipes are still lead or copper pipes, containing the components of the waste solution after the in vitro diagnostic reagent test will react with the pipes to form a very dangerous azide * lead, foreign customers also generally want to use alternatives, after years of screening and practice in the IVD industry at home and abroad, the two most commonly used in vitro diagnostic Inhibitors are organic formulation of isothiazolinone inhibitor (3% isothiazolinone dissolved in modified propylene glycol) and organic formulation of bromoxynil dioxane (10% bromoxynil dioxane dissolved in modified propylene glycol).
In vitro diagnostic bacterial inhibitors require a small addition, good bacterial inhibition, no absorption peaks at sensitive wavelengths, compatibility with various chemical components such as surfactants, while not affecting the activity of antibodies and enzymes, the product itself should be stable and can provide long-term bacterial protection against in vitro diagnostic reagents, and also have a good pH application range, finding a suitable bacterial inhibitor is not easy.
The main foreign manufacturer of isothiazolinone bacteriostatic agent is SIGMA company with the trade name ProClin 300. domestic company also successfully developed a similar product in 2015 and has been widely used in the in vitro diagnostic industry and has been exported in large quantities to the European and American markets, the product trade name NiuSha NeoCide PC-300 in vitro diagnostic reagents special antibacterial agent.
The main active ingredient of 3% isothiazolinone (hereinafter referred to as PC-300) is a mixture of two isothiazolinones, including methylchloroisothiazolinone (5-Chloro-2-methyl-4-isothiazolin-3-one, CMIT) and methylisothiazolinone (2-Methyl-4-isothiazolin-3- The product composition contains stabilizer (Alkyl carboxylate) and solvent (Modified glycol). It is suitable for PH range: 2-8.5, mainly used for nucleic acid detection reagents, immune reagents, coagulation and some biochemical reagents.
Bronidox-10% was first developed by the German company Cognis (now acquired by BASF) under the trade name of Bronidox-L, and was initially used mainly by European in vitro diagnostic companies, with 5-bromo-5-nitro-1,3-dioxane as the main active ingredient. Because of its reliable antibacterial effect, the product soon became universally accepted worldwide. It can be used alone or in combination with PC-300, which has a synergistic effect, and is mainly used in various immuno reagents, nucleic acid reagents and blood cell analysis reagents.
Controlled materials: such as sodium azide*, is a restricted and controlled product, to have a special dangerous goods warehouse, need to pass the record of explosive to purchase, each transport requires a separate application, this compliance process can not be ignored. During the epidemic, the procurement cycle will be longer. Another is the record is successful, because it is dangerous goods, spend time and energy and other management costs are also very high, coupled with the purchase, transportation, storage, use of all kinds of rules and regulations, for rapid mass production is a very unfavorable condition.
Supply and cost risks: the use of imported brands of bacterial inhibitors, will encounter is the price and delivery date, March 23, 2022, in an imported brand's official website to look at their prices and delivery date, basically in about 1-2 months, the long delivery date will face a variety of uncertainties. New crown nucleic acid detection reagents, virus preservation tube, we all certainly remember the high price from the listing to the final continuous collection of price reductions, so the antigen self-test will also face the cost of the collection of the problem, the cost of the competition behind the manufacturers will be self-evident.

Pipe product substitution
Such as sodium azide * can use other preservatives for substitution (specific alternative can be seen below the specific use of the proposed program), avoiding sodium azide * purchase, transportation, storage, use of various restrictions, once and for all.
Domestic alternative to imports
Domestic use of imported brands of preservatives, if once the surge in demand, coupled with the long import time cycle and other uncertainties, will bring huge challenges to the supply chain of downstream reagent manufacturers. At present, it is understood that domestic manufacturers are temporarily stable supply, after understanding the current domestic manufacturers of self-testing antigen reagents that have obtained a registration certificate, more than half of them have started or are prepared to use the preservatives of domestic companies for production, and individual manufacturers using sodium azide* are also in the second formulation verification at the same time, in order to reduce the subsequent supply chain risks.
High pH reagent
If the pH value of the reagent bottle > 8.5, it is recommended not to use PC-300 and BND-10, because the high pH conditions, PC-300 over time, the main active ingredient CMIT activity will decline, the effect of bacterial inhibition will have the risk of loss of control, BND-10 will also be decomposed in the high pH solution. pH > 8.5 recommended to choose NiuSha PC-950 bacterial inhibitor and NiuSha BIT- 10 bacterial inhibitor.
Each manufacturer's self-testing antigen reagent formula and content will be somewhat different, will also encounter the use of PC-300 alone, the effect of bacterial inhibition is not satisfactory, you can use Niu Sha PC-300 bacterial inhibitor + Niu Sha BND-10 bacterial inhibitor combination, dual inhibition system can obviously enhance the effect of bacterial inhibition, to achieve the purpose of bacterial inhibition. The following results of this experiment can be seen, increase the use of PC-300 alone, rather than the use of BND-10 and PC-300 combination will be more effective.
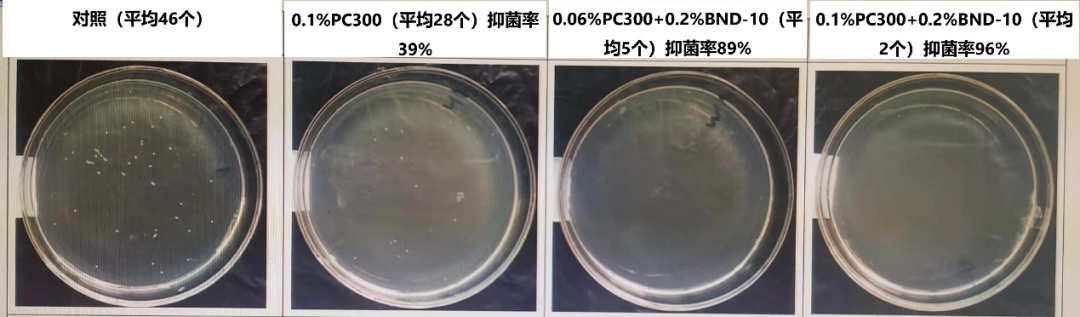
The antibacterial effect of preservatives needs to consider factors: room temperature or refrigerated storage, whether the transportation is high temperature (such as export), the expiration date (the expiration date of the reagent itself and the expiration date of the opened bottle), the complexity of the components, the environment of use, and the habits of the operator.
For self-test reagents, because it is a self-test, many people can not do according to the operating specifications, the use of the environment, the storage environment is a lot of uncertainty. Therefore, it is recommended to use double inhibition system will be a better choice: A) reagent pH less than 8.5, it is recommended to use 0.1% NiuSha PC-300 + 0.2% NiuSha BND-10 combination; B) reagent pH greater than 8.5, it is recommended to use 0.1% NiuSha PC-950 + 0.1% NiuSha BIT-10 combination, specific reference can be made to the following use program:
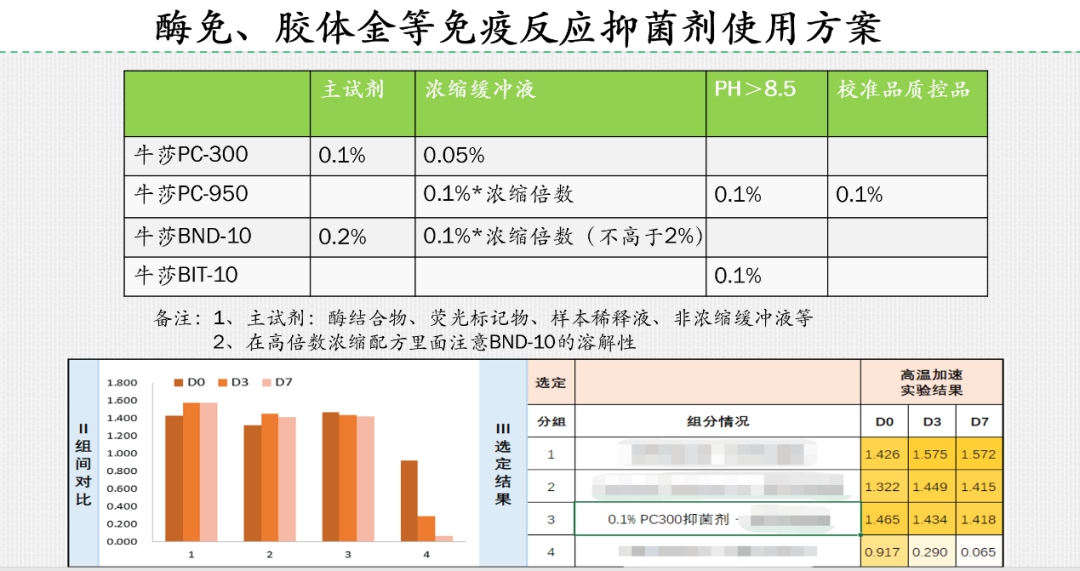
The new crown antigen self-test is currently a good opportunity and challenge, the cost control of raw materials, raw material delivery will become the focus of competition among manufacturers, in terms of preservatives, if the use of imported products, price and delivery must be considered: whether the price can be competitive under the collection? Will the production be affected due to shortage of goods? Choose some of the industry's good reputation of the national brand of preservatives to do verification, be prepared so as not to be caught off guard.